18+ Arrangement Of Electrons In Atoms Chapter 4 Review
Web Water is the chemical substance with chemical formula H 2 O. Branch of chemistry that studies isomers duh and a property of a compound to have isomers.

4 3 Pdf Name Class Date Chapter 4 Review Arrangement Of Electrons In Atoms Section 3 Short Answer Answer The Following Questions In The Space Course Hero
Web If the gate voltage is modulated from 04 to 04 V that is over 08 V an onoff ratio of 10 8 is possible.

. Web The most common way of showing the arrangement of electrons in an atom is to draw diagrams like those shown in the diagram. Web Carbon from Latin carbo coal is a chemical element with the symbol C and atomic number 6. Silicon and Germanium are elemental semiconductors and they have four valence electrons which are distributed among the outermost S and p orbitals.
The geometry of the unit cell is defined as a parallelepiped providing six lattice parameters taken as the lengths of the cell edges a b c and the angles. 116 Appendicular Muscles of the Pelvic Girdle and Lower. 115 Muscles of the Pectoral Girdle and Upper Limbs.
The atom of an element is made up of 4 protons 5 neutrons and 4 electrons. Explain why a sample of ironIII sulfate is uncharged Which of the following atoms would be expected to form negative ions in binary ionic. And octadecene C 18 H 36 found in fish liver.
18 e The number of electrons in an atom is equal to the number of. The atomic number and mass number of sodium are 11 and 23 respectively. Neutrons in a neutral atom.
To help you with it here we list a few physical and chemical properties of metals and non-metals. Web Lesson 4 Isomers and Stereochemistry Definition types and classifications 31. This arrangement is emphasized in Figure 629 which shows in periodic-table form the electron configuration of the last subshell to be filled by the Aufbau principle.
Ie the sum of R 1 R 2 and r the distance between the surfaces. 113 Axial Muscles of the Head Neck and Back. The two Chlorine atoms take one electron each thus gaining a charge of -1 each and attain the electronic configuration of the nearest.
High Order Thinking Skills. Nucleons in a neutral atom. The unit cell is defined as the smallest repeating unit having the full symmetry of the crystal structure.
The correct option is d 62510 18 electrons. There are 62510 18 electrons in one coulomb charge. Web The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing.
Stereochemistry the branch of chemistry concerned with the three- dimensional arrangement of atoms and molecules and the effect of this on chemical reactions. 203 Pauli looked for an explanation for these numbers which were at first only empirical. Web Study with Quizlet and memorize flashcards containing terms like Does a cation gain protons to form a positive charge or does it lose electrons IronIII sulfate Fe2SO43 is composed of Fe3 and 24SO ions.
Web where A is the Hamaker coefficient which is a constant 10 19 10 20 J that depends on the material properties it can be positive or negative in sign depending on the intervening medium and z is the center-to-center distance. The van der Waals force between two spheres of. D 62510 18 electrons.
Mass number Number of protons Number of neutrons 4 5 9. Web Crystal structure is described in terms of the geometry of arrangement of particles in the unit cells. Protons in a neutral atom f The sum of the number of protons and the number of neutrons present in the nucleus of an atom is.
In Chapter 1 Organic Chemistry ReviewHydrocarbons through Chapter 5 Amines and Amides we survey organic chemistry by dividing its compounds into families based on functional groups. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Web Valence electrons located on an atoms outermost shell affect how an atom will behave with other atoms.
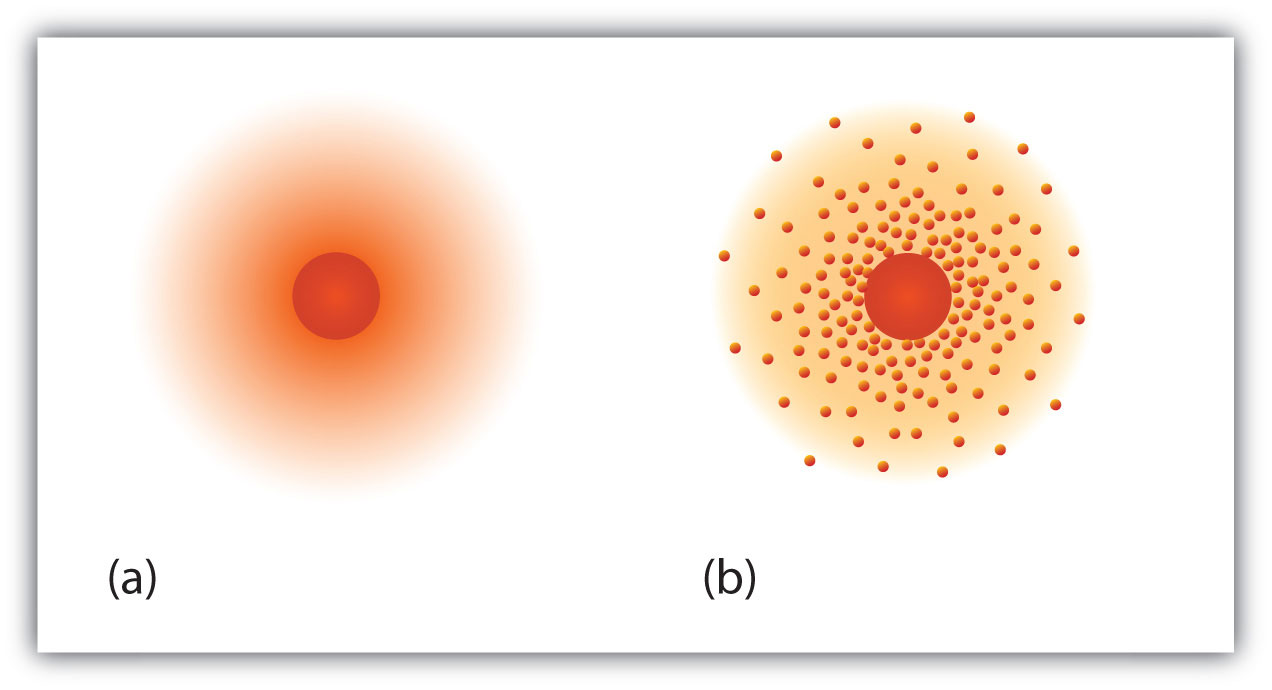
Web In atomic physics the Bohr model or RutherfordBohr model presented by Niels Bohr and Ernest Rutherford in 1913 is a system consisting of a small dense nucleus surrounded by orbiting electronssimilar to the structure of the Solar System but with attraction provided by electrostatic forces in place of gravityIt came after the solar system Joseph Larmor. C 26510 18 electrons. 112 Naming Skeletal Muscles.
Web Now we can understand why the periodic table has the arrangement it hasthe arrangement puts elements whose atoms have the same number of valence electrons in the same group. Learn more about a valence electron including its configuration and examples. Web Class 8 Science Chapter 4 discusses the various physical and chemical properties of metals and non-metals.
On-state current densities of 1 mA μm 1 Fig. Web The resulting molecules can contain from one to millions of carbon atoms. Web In chemistry and manufacturing electrolysis is a technique that uses direct electric current DC to drive an otherwise non-spontaneous chemical reaction.
Web Groups of electrons were thought to occupy a set of electron shells around the nucleus. Atomic number Number of protons or number of electrons 4. What are its atomic number and mass number.
Web Ultraviolet UV is a form of electromagnetic radiation with wavelength from 10 nm with a corresponding frequency around 30 PHz to 400 nm 750 THz shorter than that of visible light but longer than X-raysUV radiation is present in sunlight and constitutes about 10 of the total electromagnetic radiation output from the SunIt is also produced by electric arcs. This allows the smaller carbon atoms to enter the interstices of the iron crystal. Three 2V cells are connected in series and used as battery in a circuit.
A What is the pd at the terminals of the battery. These outer most S and p orbitals of Semiconductors involve in Sp3 hybridanisation. Three isotopes occur naturally 12 C and 13 C being.
Web For example calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. For example visual experience appears to have a structure that corresponds to the spatial environment being experienced and experience as a whole comes carved up into distinct sensory modalities. 111 Interactions of Skeletal Muscles Their Fascicle Arrangement and Their Lever Systems.
When a Ca atom loses both of its valence electrons the result is a cation with 18 electrons a 2 charge and an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6. None of the above. In 1922 Niels Bohr updated his model of the atom by assuming that certain numbers of electrons for example 2 8 and 18 corresponded to stable closed shells.
3ab are achievable. These Sp3 orbitals form four covalent bonds of equal angular separation leading to a tetrahedral. For example the atomic number of carbon is 6 giving us six electrons as 24.
114 Axial Muscles of the Abdominal Wall and Thorax. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. To write down the numbers of electrons in each energy level.
Web 442 The Structural Mismatch Problem Human conscious experience is not only rich in qualities but also rich in structure. Carbon makes up only about 0025 percent of Earths crust. The atomic number of an element tells us how many electrons there are in the atoms.
Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cellThe voltage that is needed for electrolysis to occur is. It is nonmetallic and tetravalentits atom making four electrons available to form covalent chemical bondsIt belongs to group 14 of the periodic table. Web b 6210 19 electrons.
Web The base metal iron of the iron-carbon alloy known as steel undergoes a change in the arrangement of the atoms of its crystal matrix at a certain temperature usually between 1500 F 820 C and 1600 F 870 C depending on carbon content. Protons in a neutral atom.
Full Article Photoionisation Of Ions With Synchrotron Radiation From Ions In Space To Atoms In Cages

Pdf Advices For Studying Organic Chemistry Sabrina Islam Academia Edu
High Resolution Momentum Imaging From Stern S Molecular Beam Method To The Coltrims Reaction Microscope Springerlink

Electrons In Atoms 5 North Plainfield School District Guset User Flip Pdf Anyflip
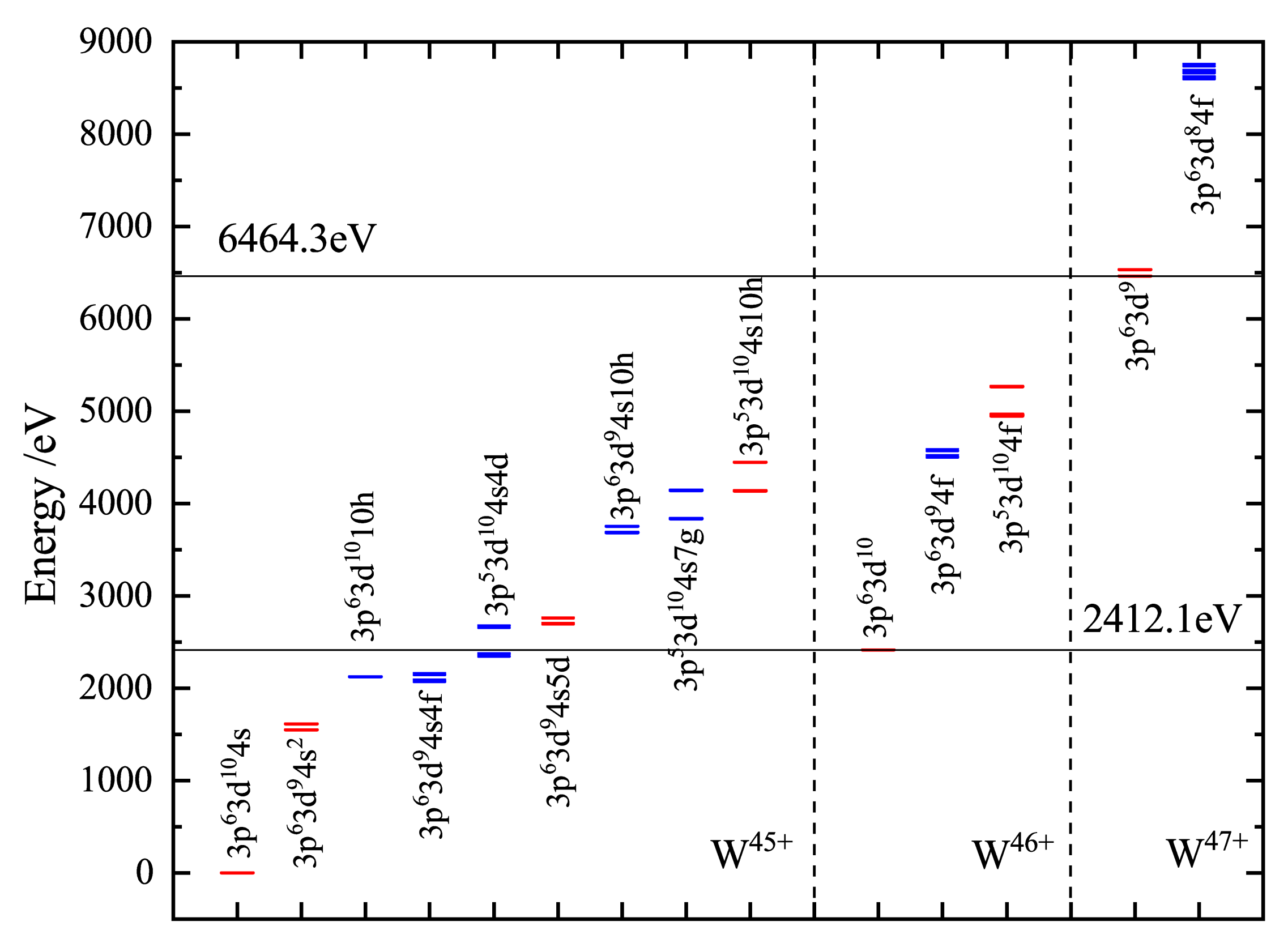
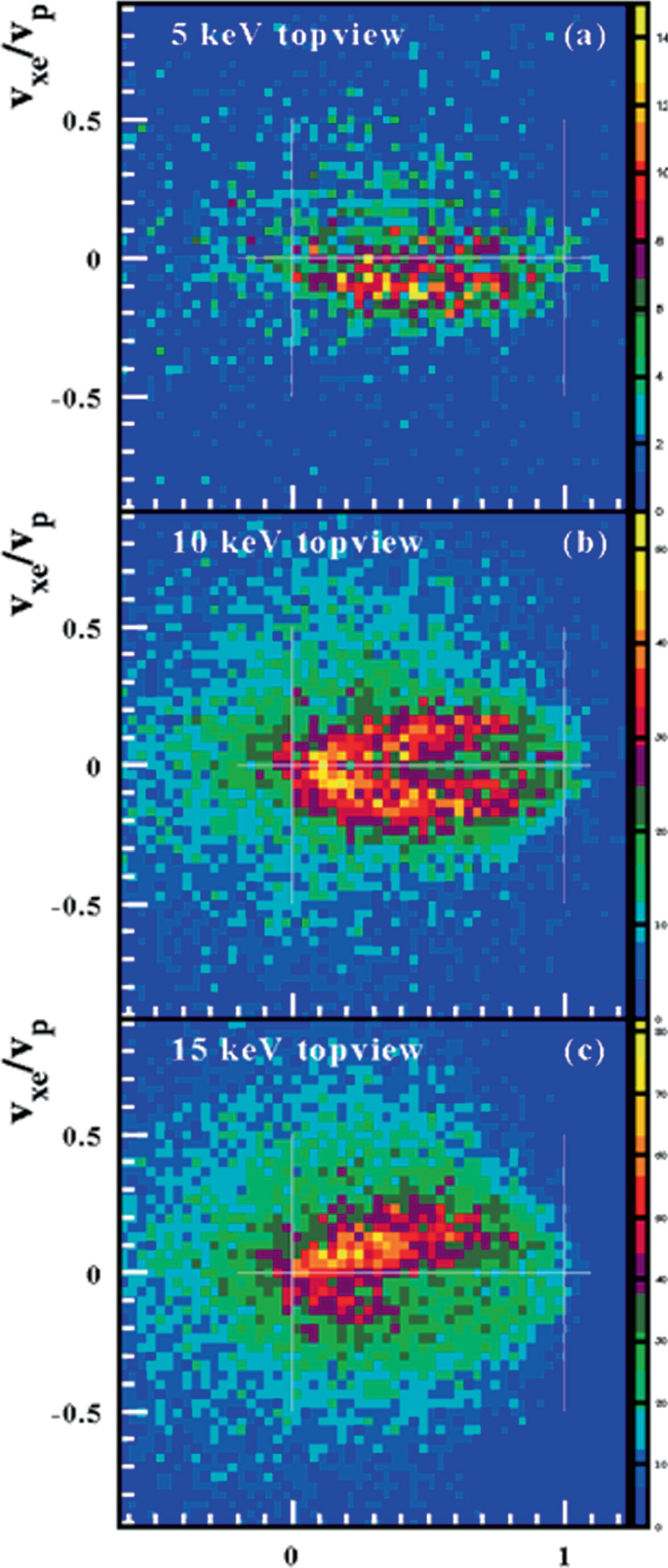
Atoms Free Full Text Electron Impact Ionization Of The Tungsten Ions W38 Minus W45
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
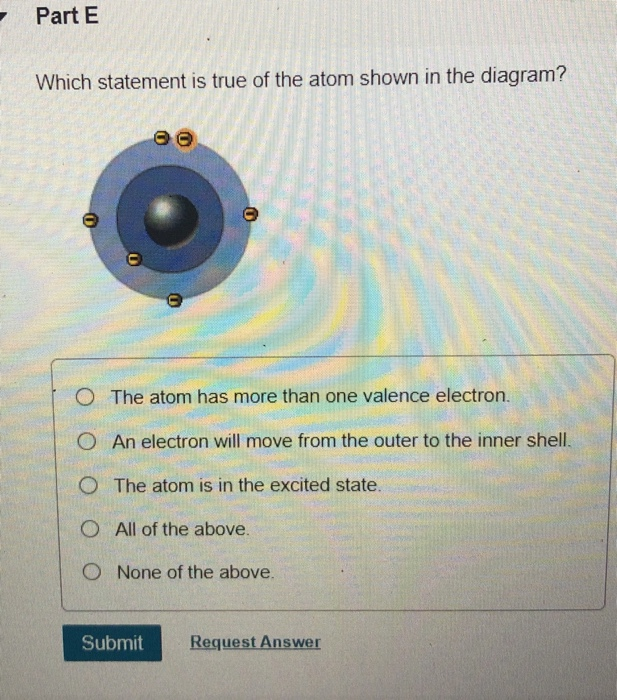
Solved A B D E Which Drawing In The Figure Depicts The Chegg Com
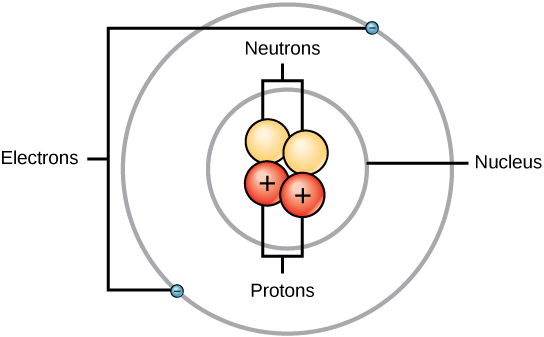
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

Uncovering Multiple Metal Metal Bonding In A Tetranuclear Fluoride Rhenium Cluster Or The Curious Case Of Ni H2o 6 Nh4 4 Re4f18 4h2o Mariappan Balasekaran 2019 European Journal Of Inorganic Chemistry Wiley Online Library

Arrangement Of Electrons In Atoms Pdf Free Download

Which Of The Following Have An 18 2 Electron Configuration

Atomic Structure

Arrangement Of Electrons In Atoms Pdf Free Download

Electron Transfer Through 3d Monolayers On Au25 Clusters Acs Nano

Ion Beam Science Solved And Unsolved Problems

Chemistry Chapter 4 Arrangement Of Electrons In Atoms Ppt Download
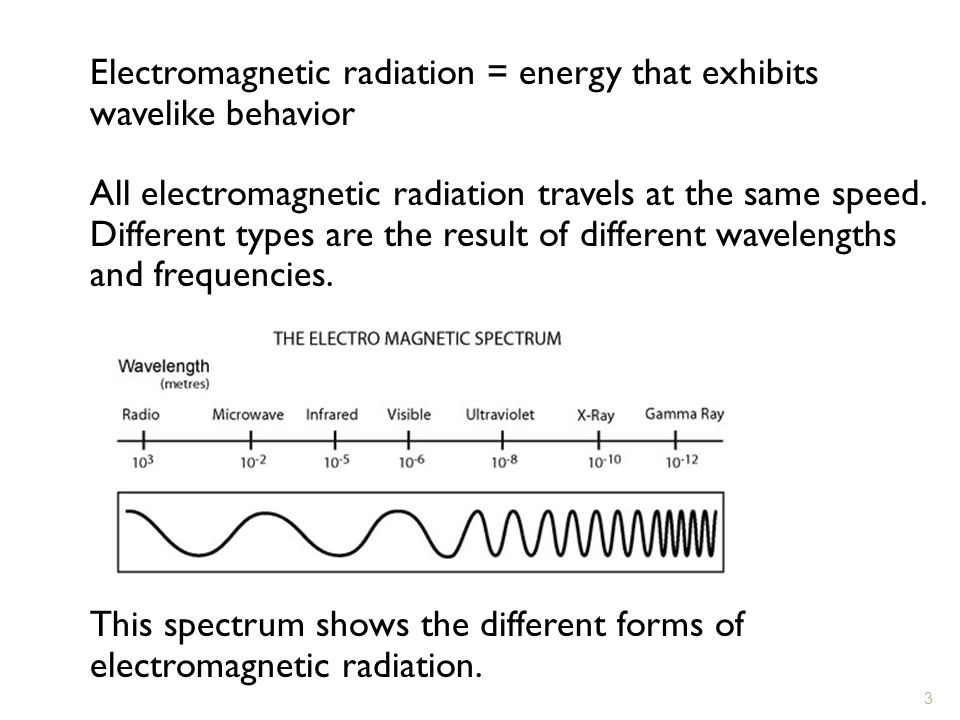
Chapter 4 Arrangement Of Electrons In Atoms Ppt Video Online Download